About My Blog & Mission
Hi, this is my personal blog. Here, I highlight research articles published in various (polymer based)journals (of Nature Portfolio, Royal Society of Chemistry, American Chemical Society, Cell Press, Elsevier, Wiley, MDPI, AAAS, etc.) under the theme "Plastic Pollution & Mitigation Strategies".
Currently, we are living in a world where our material demands and unsustainable consumption patterns (linear economy – take, make, use and disposal) have caused major environmental consequences such as plastic pollution (both on land & in oceans), climate change (global warming) due to greenhouse gas emissions, and depletion of finite fossil resources – which are imposing a threat to our planet!
We need sustainable solutions that are restorative and regenerative, energy efficient technologies, and transformation of chemical/plastic/material industry from a linear (take–make–use–disposal) to a circular economy (take–make–use–recycle/upcycle) must take place! – ultimately these will contribute to achieve most of the United Nations Sustainable Development Goals outlined in agenda 2030 {such as SDG 6: clean water & sanitation; SDG 7: affordable & clean energy; SDG 9: industry, innovation & infrastructure; SDG 11: sustainable cities & communities; SDG 12: responsible consumption & production; SDG 13-15: climate action, life below water & life on land} as well as the common goal of limiting global warming to below 2 degree celsius as per Paris Agreement.
To tackle the abovementioned challenges, researchers in both academia and industry are engaged in finding innovative solutions – by re-thinking and re-designing of the whole plastic production value chain – that utilize abundant renewable feedstocks (biomass, plastic waste, carbon dioxide), renewable energy (solar, wind) and non-hazardous chemicals (benign solvents such as deep eutectic solvents or solventless methods, replacements for endocrine disruptors such as bisphenol-A) with an emphasis on end-of-life (recycling/upcycling).
"My blog mission here is to promote and bring more visibility to those ongoing academic efforts, especially those accelerating toward the Circular Economy Transition – which is imperative for eliminating current plastic pollution and to achieve a sustainable society"
I cover mainly the following topics:
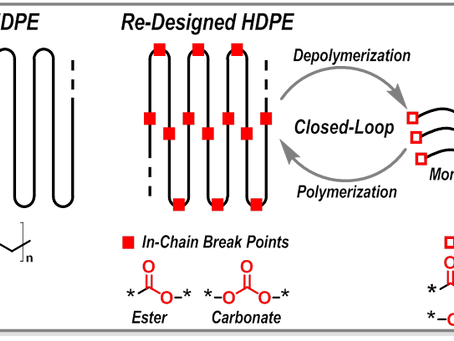
(1) Design of new polymers with closed-loop life cycles – which is considered as an ideal solution to achieve a sustainable 'Circular Plastic Economy' by the research experts in the polymer community. Because they can be chemically depolymerized back into their starting monomers 'infinite times' ('monomer–polymer' closed-loop life cycles), they provide pristine-like quality material properties after each recycling loop, and thereby high monetary value can be achieved, as opposed to current industrial practice of mechanical recycling (grinding-melting-pelletizing), in which polymer chain undergoes scission/degradation (with each recycling loop) and thereby deterioration in material properties (decrease in molecular weight & thermo-mechanical properties) and their (re)use is limited to low grade applications (for example, recycling of PET bottles to carpet making, and further recycling of such mixed materials is difficult).
Polymers with closed-loop life cycles are mainly those containing cleavable units such as esters, carbonates, amides, acetals, thioesters, etc., on the polymer backbone – re-designing is mainly focused on the development of polymers that can mimic the properties of polyolefins or other widely used existing polymers in our day-to-day life. To this end, innovations based on both thermoplastic and thermosetting based polymers are covered. For example, in case of thermoplastic polymers, sustainable polymers with closed-loop life cycles obtained either via ring-opening polymerization of five to eleven membered cyclic monomers (that is, intrinsically circular polymers with low ceiling temperature) or via condensation polymerization by utilizing end-group monomers (hydroxyl/acid or methyl ester groups) or via incorporation of cleavable additive monomers during free-radical polymerization will be highlighted (few examples are already available here on my blog, see Research Highlights – 2, 8, 9 & 10).
In case of thermosetting resins, innovations based on 'vitrimers', that is covalent adaptable networks with an associative rearrangement mechanism will be highlighted (which exhibit 'polymer–polymer' closed-loop life cycles). Vitrimers are permanently cross-linked polymer networks, but unlike traditional thermosetting polymers, they can be thermally reprocessed/recycled/reshaped due to the presence of dynamic bonds (few examples: ester, imine, vinylogous urethane, diketoenamine, acetal, etc.) in the network. They undergo an associative type exchange reaction (addition-elimination pathway), either with an active group {for example, transesterification of esters with hydroxyl groups or transamination of imines with amine groups} or shuffling b/w dynamic bonds {for example, boronic ester metathesis, imine metathesis, acetal metathesis} in the network which allows macroscopic flow. Since the bonds are only broken after the formation of new ones through a short-lived associative intermediate (meaning no depolymerization), the crosslink density of the network remains constant throughout the exchange reaction, as a result they fully retain thermo-mechanical properties (virgin-like) after multiple reprocessing cycles – which is not the case during traditional mechanical recycling of thermoplastics. Hence, vitrimers are considered as a sustainable alternative for both traditional thermosets and thermoplastics. Moreover, dissolution is possible in case of vitrimers (similar to thermoplastics) by addition of an excess monofunctional monomer in a suitable solvent at a high temperature – for example, addition of an excess mono-amine in a vinylogous urethane vitrimer can disrupt the network via transamination, which displaces di/tri-amine crosslinker in the network with mono amines via exchange between mono amines and vinylogous urethane bonds leading to dissolution – and the network can be regained by balancing the stoichiometry using original bi/tri-functional monomer (Sumerlin et al, Macromolecules, 2019, 52, 2105). Thus, vitrimers allow closed-loop recycling of fiber-reinforced plastic (FRP) composites (that is recycling of all materials in FRP: matrix resin, fibers and any fillers used), which is not possible with traditional thermosetting resin as they cannot be solubilized by using heat or solvents due to their permanent covalently crosslinked three-dimensional network structure.
Circular polymers that possess both infinite chemical recyclability ('monomer–polymer' closed-loop life cycles) and vitrimer-like properties ('polymer–polymer' closed-loop life cycles) are also a topic of interest as they can be recycled by using either chemical recycling or thermal reprocessing depending on demand, at the same time will be able to provide pristine-quality material properties (an example of such circular polymer is 'polydiketoenamine' invented by Helms et al, see Research Highlight 5 on my blog). Academic innovations on these topics can definitely contribute to eliminate plastic pollution, and thus will be prioritized for highlighting here on my blog.
(2) Post-consumer plastic waste recycling/upcycling – either into its starting monomers to ‘close the loop’ or high value chemicals/materials. The latter called 'open-loop life cycle' as a means to fully utilize the embedded material in a waste via upgrading it to similar or high value chemical/polymer, so that it does not enter into the waste stream and instead circulate in the economy. Ongoing academic innovations on both lines will be highlighted here on my blog. Emphasis will be devoted to plastics that are widely used in packaging applications, that is the Society of the Plastic Industry (SPI) Codes 1-7: polyethylene terephthalate [PET, SPI Code 1], polyethylene (PE): high-density PE [SPI Code 2] & low-density PE [SPI Code 4], polypropylene [PP, SPI Code 5], polystyrene [PS, SPI Code 6], polyvinyl chloride [PVC, SPI Code 3], & other [SPI Code 7: polycarbonate (PC), polyamide (PA), polyurethane (PU) and so on]. Majority of the plastic waste in the municipal solid waste stream accounted to these polymers and most of them are disposed of after a single-use application. Hence, in my opinion, bringing more visibility to the academic innovations that can circulate these plastics in the economy will inevitably contributes to eliminate plastic pollution and accelerate the circular economy transition of the plastic industry. To this end, emphasis will be on the following topical areas:
(i) New catalytic depolymerization methods for recycling or upcycling of post-consumer plastic waste [SPI Codes 1-7] into feedstock monomers/fuels/lubricants/waxes. Few methods are listed here:
-
By means of solvolysis
{hydrolysis, alcoholysis, aminolysis, glycolysis, etc.} – these depolymerization strategies are only pertinent to polymers containing cleavable or hydrolyzable functional groups. A published example is upcycling of bisphenol-A polycarbonate (BPA-PC) into high value five/six-membered cyclic carbonate monomers by using 1,2-/1,3-diols and TBD:MSA protic ionic salt catalyst – this strategy recovers both carbonate and bisphenol structural part during depolymerization as opposed to the state-of-the-art hydrolysis of BPA-PC in which only bisphenol-A is recovered, and the carbonate part is lost in the process as carbon dioxide (Sardon & Dove et al, ACS Macro Letters, 2020, 9, 44). Such innovative works will be highlighted.
-
Hydrogenolysis – that is, depolymerization of polymer over a dehydrogenation/hydrogenation metal catalyst under hydrogen pressure. Academic efforts are mainly focused on hydrogenolysis of polyolefins to selectively produce high value liquid hydrocarbons in the range of gasoline [C5-C12], jet fuel [C8-C16], diesel [C9-C22] and wax/lubricating oil [C20-C35]). During polyolefin hydrogenolysis, dehydrogenation occurs at first via C-H activation over the metal catalyst (for example over Ru/C) which leads to cleavage of C-C bond to form olefin intermediates, followed by hydrogenation and desorption of these intermediates to form the above-mentioned liquid hydrocarbons. However, the main problem is the rate-limiting hydrogenation/desorption step, and thus the degraded intermediates undergo multiple dehydrogenation and C-C bond cleavage steps (instead of hydrogenation/desorption) to produce low value gases such as methane. Moreover, high hydrogen pressure is generally required for cleavage of internal C-C bonds, otherwise leading to methane formation via terminal C-C bond cleavage. Thus, efficient catalysts are needed to suppress methane formation and facilitate the hydrogenation/desorption step and produce high value liquid fuels without needing to use high hydrogen pressure. Academic innovations on this front will be highlighted. A published example is hydrogenolysis of real-world single-use LDPE plastic wastes such as bottles, cling wraps & laboratory pipettes into liquid fuels (C9-C35 range) over ruthenium supported on tungstated zirconia (Ru-15WZr) under mild conditions (hydrogen pressure of 50 bar and at 250 degree). This catalyst stores spill-over hydrogen dissociated from Ru at WOx surface sites (that is, at tungstate) and accelerates the hydrogenation/desorption step by supplying stored active hydrogen (Vlachos et al, JACS Au, 2021, 1, 1422). Hydrocracking of polyolefins will also be highlighted here, which also depolymerizes the polymer under hydrogen pressure, but over a bifunctional catalyst consisting both metal and acid sites (that is, dehydrogenation/hydrogenation metal catalyst & solid acidic zeolites). The main difference lies in the mechanism and also in the selectivity range of the obtained liquid fuels. During hydrocracking, the C-H activation for dehydrogenation occurs at the metal site, however unlike hydrogenolysis, the cleavage of the C-C bond occurs at the Bronsted acid sites via carbocation intermediate formation, which follows isomerization, and then diffusion of these olefin intermediates onto metal sites for hydrogenation to produce liquid hydrocarbons. A published example is hydrocracking of real world plastics such as HDPE bottles, LDPE bottles, HDPE single-use transparent bags and composite tapes consisting a blend of 45% PP, 45% PE & 10% PS into a mixture of gasoline, jet and diesel range fuels over a bifunctional catalyst platinum supported on tungstated zirconia and HY zeolite (Pt/WO3/ZrO2 + HY-30) under mild conditions (hydrogen pressure of 30 bar and at 250 degree) (Vlachos et al, Science Advances, 2021, 7, eabf8283). Here, dehydrogenation/hydrogenation occurs at the Pt sites, C-C cleavage at the acid sites of WO3/ZrO2 and HY-zeolite and isomerization at the WO3/ZrO2 sites. Innovations related to novel bifunctional catalysts that can selectively produce narrow distributed high value heavy liquid fuels, for example diesel range, by tuning the catalyst composition (metal-acid balance) and under mild conditions (low temperature & hydrogen pressure) will be highlighted. Other than polyolefin hydrogenolysis, innovations based on hydrogenolysis/hydrocracking of plastic containing polar bonds (C-O & C-N) will also be highlighted. A published example is conversion of aromatic plastic wastes such as PC, PET, PS and polyphenylene oxide (PPO) into 'arenes' using a multifunctional Ru/Nb2O5 catalyst under hydrogen pressure. Here, the sub-nanosized clusters of Ru on Nb2O5 prevent over hydrogenation (they prevent co-adsorption of hydrogen and benzene) and preserve the aromatic structure of the polymer to produce arenes. The selective cleavage of interunit C-O/C-C bonds in the polymer backbone was achieved by activation at the Lewis acid and Bronsted acid sites of NbOx and by the involvement of dissociated hydrogen over Ru. This catalyst can also selectively produce a single high-value chemical, for example m-xylene was produced in quantitative yield from PPO (Yan et al, Angewandte Chemie International Edition, 2021, 60, 5527). Another excellent published example is the utilization of hydrogenation/dehydrogenation process to 'close-the-loop' of nylons (Milstein et al, Journal of the American Chemical Society, 2020, 142, 14267). Here, hydrogenative depolymerization of nylon-6 over a ruthenium pincer catalyst under hydrogen pressure (at 150 degree in DMSO and potassium tert-butoxide base) results in amino alcohol monomer (that is, 6-amino-1-hexanol) via cleavage of C-N bonds along with some oligoamides (containing hydroxyl & amine end groups). Nylon-6 can be recovered by utilizing these depolymerized monomer + oligomers via dehydrogenative coupling in the presence of a ruthenium pincer catalyst complex. Such innovative works will be highlighted here on my blog.
-
Cascade reactions – a published example applied for PE is tandem catalytic cross alkane metathesis (CAM) to depolymerize real-world plastic wastes such as HDPE bottles, HDPE food packaging film and a grocery bag made from a blend of HDPE & LLDPE into fuels/waxes by using bis(phosphinite)based (POCOP) iridium complex dehydrogenation/hydrogenation catalyst and Re2O7/Al2O3 metathesis catalyst in the presence of short chain alkanes such as petroleum ether/n-hexane/n-octane (which act as both solvent and metathesis partner). In this strategy, at first PE and the short alkane undergo dehydrogenation to form unsaturated PE and olefin, and then cross-metathesis takes place between these to cleave the PE backbone, followed by hydrogenation of the degraded olefin intermediates to form long-chain alkanes, and this tandem CAM cycle repeats to produce high-value liquid fuels in the range of 'diesel' along with a small amount of wax (Huang et al. Science Advances, 2016, 2, e1501591). Academic innovations that utilize such cascade reactions for recycling/upcycling of plastic wastes [SPI Codes 1-7] will be highlighted especially those producing feedstock monomers.
-
(photo) Oxidation – plastic wastes based on both hydrolyzable & non-hydrolyzable plastics [SPI Codes 1-7] will be highlighted. For example, oxidation of PET to terephthalic acid, polyolefins to aliphatic (mono/di)carboxylic acids, PS to benzoic acid and so on. An example is already highlighted here on my blog as Research Highlight 6 – chemical recycling of real-world PS plastic wastes such as takeaway coffee cups/food boxes & yogurt containers into benzoic acid via acid-catalyzed aerobic oxidation under visible light – a work by Xiao et al. Apart from that, fenton reaction, that is, Fe cations mediated reactive oxygen species generation for the decomposition and mineralization of plastic waste into high value chemicals, carbon dioxide & water will also be covered for highlighting.
-
Biotechnological conversion using enzymes and microorganisms – an excellent example is already highlighted here on my blog as Research Highlight 1 – biotechnological conversion of PET bottles into vanillin – a work by Wallace et al. Here, the hydrolytic breakdown of PET into terephthalic acid (TPA) occurs in the presence of Leaf-branch Compost Cutinase (LCC) enzyme, followed by de-novo conversion of TPA into vanillin using Escherichia Coli (E. coli) – MG1655 RARE (Reduced Aromatic Aldehyde Reduction) as the host microorganism. Other than that, special focus will be devoted for highlighting the discovery of new enzymes for bioconversion of non-hydrolyzable plastics (that is, SPI Codes 2-6) into feedstock monomers/high value chemicals. Recently published examples are: biooxidative depolymerization of PE into ketones using Wax Worm Saliva enzymes 'Demetra and Ceres phenol oxidases' (Bertocchini et al, Nature Communications, 2022, 13, 5568) and bioconversion of PS into acrylic acid and 2-hydroxypenta-2,4-dienoate using Exiguobacterium sp. RIT 594 – here, the depolymerized monomer styrene undergoes hydroxylation and aromatic ring cleavage by a dioxygenase enzyme, and then further degradation by a hydrolase enzyme to produce the aforesaid compounds (Hudson et al, Microorganisms, 2022, 10, 1619).
-
External hydrogen source-free plastic conversion technologies – a published example is (Wang et al, ChemSusChem, 2021, 14, 4242) conversion of PET into drop-in chemicals BTX [benzene, toluene & xylene] via unlocking structurally hidden hydrogen in one of the depolymerized monomers, ethylene glycol. In this strategy, PET undergoes tandem reactions over Ru/Nb2O5 catalyst – at first hydrolysis to produce terephthalic acid and ethylene glycol, and then ethylene glycol re-forming with water to generate in-situ hydrogen, which then participates in hydrogenolysis and decarboxylation of terephthalic acid to produce BTX.
-
Solar assisted plastic recycling technologies – one strategy is photoreforming of plastic waste into hydrogen – which entails a combination of reactions that involve photooxidation of the substrate into organics and splitting of water into hydrogen under the presence of a photocatalyst and sunlight. A published example is (Reisner et al, Energy & Environmental Science, 2018, 11, 2853) photoreforming of PET, PU and polylactide into hydrogen over CdS/CdOx quantum dots in alkaline media and under simulated sunlight. For example in case of PET, it undergoes hydrolysis in alkaline aqueous media to produce terephthalate and ethylene glycol, and the latter the aliphatic component act as substrate for hydrogen production (electron donor) and photooxidized into organic molecules (such as formate, lactate, acetate, glycolate and ethanol) and carbon dioxide, whereas terephthalate can be isolated in its salt form. Second strategy is photocatalytic conversion of plastic waste into carbon dioxide. Here emphasis will be on highlighting academic innovations that probe their further conversion, for example hydrogenation or reduction of carbon dioxide by reaction with hydrogen or water respectively to produce C1- (methanol, formic acid) and/or C2-fuels (acetic acid, olefins, ethanol, ethylene glycol, etc.). A published example is (Xie et al, Angewandte Chemie International Edition, 2020, 59, 15497) photocatalytic conversion of real world plastic wastes such as single use bags (PE), disposable food containers (PP) and food wrap films (PVC) into carbon dioxide via photooxidative C-C cleavage and followed by photoreduction of carbon dioxide into acetic acid (via C-C coupling of COOH radical intermediates) using single-unit-cell thick NbO5 layers as photocatalyst and under sunlight irradiation. Academic innovations that couple photoreforming with C1-/C2-fuels production are currently lacking, in the current state-of the art photoreforming is mainly focused on hydrogen production, photocatalysts that can fully mineralize plastics (that is conversion of organic molecules produced) into carbon dioxide are needed to further utilize this strategy for C1/C2 fuels production – innovations in this research direction will be highlighted. These solar assisted catalytic strategies are not only useful for upcycling of well-sorted and a single stream plastic waste into renewable hydrogen and fuels, but also a remedy for dealing with non-recoverable plastic wastes which were previously destined for landfilling or incineration such as highly food contaminated plastic wastes, textile fibers, and also microplastics – thus will be prioritized for highlighting here on my blog.
(ii) Strategies for chemical recycling of multi-stream plastic waste – new catalysts and methodologies [including all those listed in (i)]. Currently the difficulty of sorting post consumer plastic wastes is the main hindrance to their recycling and most of them end up in landfill or incinerated. Even though, sorting technologies are advancing – tracer-based sorting, integration of digital watermarks into packaging, robot sorting, and so on, we need research innovations that are capable of recycling multi-stream plastic waste to enhance the recycling rate of these plastics. An excellent example is already highlighted here on my blog as Research Highlight 7 – a new catalytic methodology 'KMH solution' (that is potassium hydroxide in methanol) by Thielemans et al, which allows chemical recycling of mixed plastic waste stream containing PC and PET into corresponding starting monomers bisphenol-A (from PC), terephthalic acid and ethylene glycol (from PET) without any side reaction, and the isolation of these monomers from the depolymerized mixture is simple and non-energy intensive. Such work is a sign that, with continued academic innovation and efforts, soon we can be in a world where all plastic wastes (for example condensation type – that is, those containing hydrolyzable groups – PET, PC, PA, PU, polyacetals, and so on) are collected in ‘one bin' and chemically depolymerized back into their starting monomers or high value chemicals using ‘one-pot and a single catalytic methodology'. Right now, It may seem highly ambitious to hear, but Prof. Thielemans's work says that it’s not impossible! Bringing visibility to such works can motivate policymakers to put right policies in place, for example to collect these abovesaid plastic wastes in one bin. Hence, definitely it is a top priority & aim of my blog to highlight such ground-breaking works! Academic innovations that can chemically depolymerize both hydrolyzable & non-hydrolyzable plastics containing mixed waste stream (for example: PE, PP, PS & PET) will also be highlighted, since this can completely avoid sorting of these plastics into different categories, and instead collect them in one-bin & depolymerize them in one-pot (for example by means of oxidation) into high value feedstock monomers!
(iii) Recycling of multi-layer flexible packaging materials (MLFP) – Innovations related to recycling of multi-layer flexible packaging films are very scarce in the literature and need contributions in this area if we really want to mitigate plastic pollution, since MLFP constitutes ~20% of plastic packaging industry (mainly food packaging: chips packets, biscuit packets, and others like shampoo sachets). Multi-layers are used in order to meet a combination of properties, for example, PET is used as an outer layer for strong and printable surface, PU as tack layers (for lamination), aluminium or polyvinylidene chloride as barrier layers (gas barrier, protection against UV light), PA is used as abrasion resistant layers and PE/PP as an inner food contact layer (for strong, heat sealing properties and moisture barrier). Currently three methodologies are employed for recycling of MLFP waste: one strategy is selective dissolution-precipitation {also known as solvent-targeted recovery and precipitation (STRAP)} of polymer layers, in which each polymer is selectively dissolved using a suitable solvent and an anti-solvent is added to recover the dissolved polymer from the solution. This process is repeated until all layers in MLFP waste are recycled. Instead of using the antisolvent, innovations such as temperature-induced precipitation (by cooling the polymer solution) especially when two different layers are soluble in the same solvent during STRAP is employed (Huber et al, ChemSusChem, 2021, 14, 1) or greener alternatives such as switchable hydrophilic solvent is used (Denafas et al, Green Chemistry, 2018, 20, 3604). There is a growing interest from industry for STRAP and many industrial pilot scale plants are already implemented (CreaSolv Sachet Recycling Plant, Indonesia). Second strategy is depolymerization of polymer layers using suitable solvents and catalysts (for example by means of solvolysis) to recover starting monomers from these polymer layers. Third strategy is the dissolution of tie layers (that is, PU adhesive) via diffusion using acids/solvents, in which polymer layers (& aluminium) are recovered without damage in shape and size. In the third scenario there are emerging innovations to replace PU with crosslinkable reversible adhesives such as those based on (retro)Diels-Alder reaction of furan/maleimide, which can be solubilized by a heated solvent (Kaiser et al, Recycling, 2021, 6, 47). Innovations based on these research lines will be highlighted here on my blog.
(iv) Upcycling of plastic wastes into covalent adaptable networks (that is, vitrimers) for improved circularity – which also impart them properties such as high melt-strength, good dimensional stability and better environmental stress cracking resistance as compared to the parent plastic. More importantly, they can be processed like traditional thermoplastics using extrusion, injection molding and 3D printing. A published example is polyolefin vitrimers obtained via upcycling of real-world plastic wastes such as HDPE packaging materials and recycled PP bottles via C-H modification of the polymer chain – that is, maleic anhydride grafting on polyolefin chain via reactive extrusion, and followed by curing with bisphenol-A based epoxy resin (DGEBA) in the presence of zinc acetylacetonate hydrate catalyst. The resulting ester groups in the crosslinked network can undergo transesterification to rearrange the topology of the network (with the same catalyst), thereby exhibiting vitrimer properties such as reprocessability, reshapability and also weldability for example PE with PP, which otherwise not possible because of their incompatible interfaces (Terentjev et al, Journal of Materials Chemistry A, 2020, 8, 24137). Such research innovations will be highlighted.
All articles from all journals that come under the broad umbrella of the abovementioned theme "Plastic Pollution & Mitigation Strategies" [(1) & (2)] (including those not listed here) are considered for 'highlighting'.
In my "Research Highlights" (blog posts), I generally highlight the author's breakthroughs, their next steps and more importantly impact of their work on societal/environmental/economic level – 'how their work is contributing to achieve a circular plastic/material economy and a sustainable society'. They connect the dots between academic innovations (current academic literature), policies and industrial needs/demands, and aimed for bringing these academic works more into light! – beyond just accelerating their industrialization or commercialization.
To accelerate the circular economy transition of plastic/chemical industry, efforts and coordination from all parties are necessary including academia (research & development innovations), government (for stringent policy regulations), industry (to inform about established material flows & implementation of new ones), and society (consumer behavior & awareness). I strongly believe that I'm doing my part "through my blog" – by promoting these ongoing academic innovations that have real potential to tackle current global challenges, and by informing about them to the stakeholders through "my Research Highlights'', which can inevitably leads to policy changes – they can motivate policymakers to put right waste management strategies/policies in place and ultimately contributing to enhance the recycling rate of the plastics and the circular economy transition of plastic/polymer industry.
My 'Research Highlights' are insightful and intended for a diverse audience – not only for academic peers, but also for non-academic audience & non-experts from a diverse community (including industrialists, policymakers & citizens). They give fresh perspectives/new ideas to the readers including to the authors of the articles highlighted, the points they might have missed to highlight, and will point the direction of the research should take. My hope is that they will serve as a force for societal changes (society wide transition of circular economy) that promise us a sustainable future!
By, Sini Nalakathu Kolanadiyil.
About Me
My research background is in Polymer Chemistry (Bachelor’s & Master’s Degree) with a specialization in (Poly)Benzoxazine Chemistry – a phenolic type thermosetting resin. I have obtained a PhD from IITD India (in 2014), and four-years of postdoctoral experience from Kindai University, Japan (2014-2018) on this topic. Currently, I’m in the process of seeking funding (+Host – EU/UK based) for my research proposal in the ‘5-year Junior Research Group Leader Fellowship Programme’ which will allow me to establish my own research group in the desired university. My research proposal targets current environmental problems caused by plastic pollution and provides adequate solutions to address them – designing of novel renewable & recyclable polymers with closed-loop life cycles for circular economy practices by utilizing biomass & plastic waste. This research blog is something I’m doing out of passion. I have dedicated my spare time for this mission.